






Novel Coronavirus (2019-nCoV) Antigen Test Kit - Nasal Swab
The applicable population should refer to the national regulations such as the "Application Plan for COVID-19 Antigen Testing (Trial)".
Classification: Novel Coronavirus (2019-nCoV) Rapid Test Kit
Hotline:400 007 9938/ 13073192811-Domestic sales
Product Description
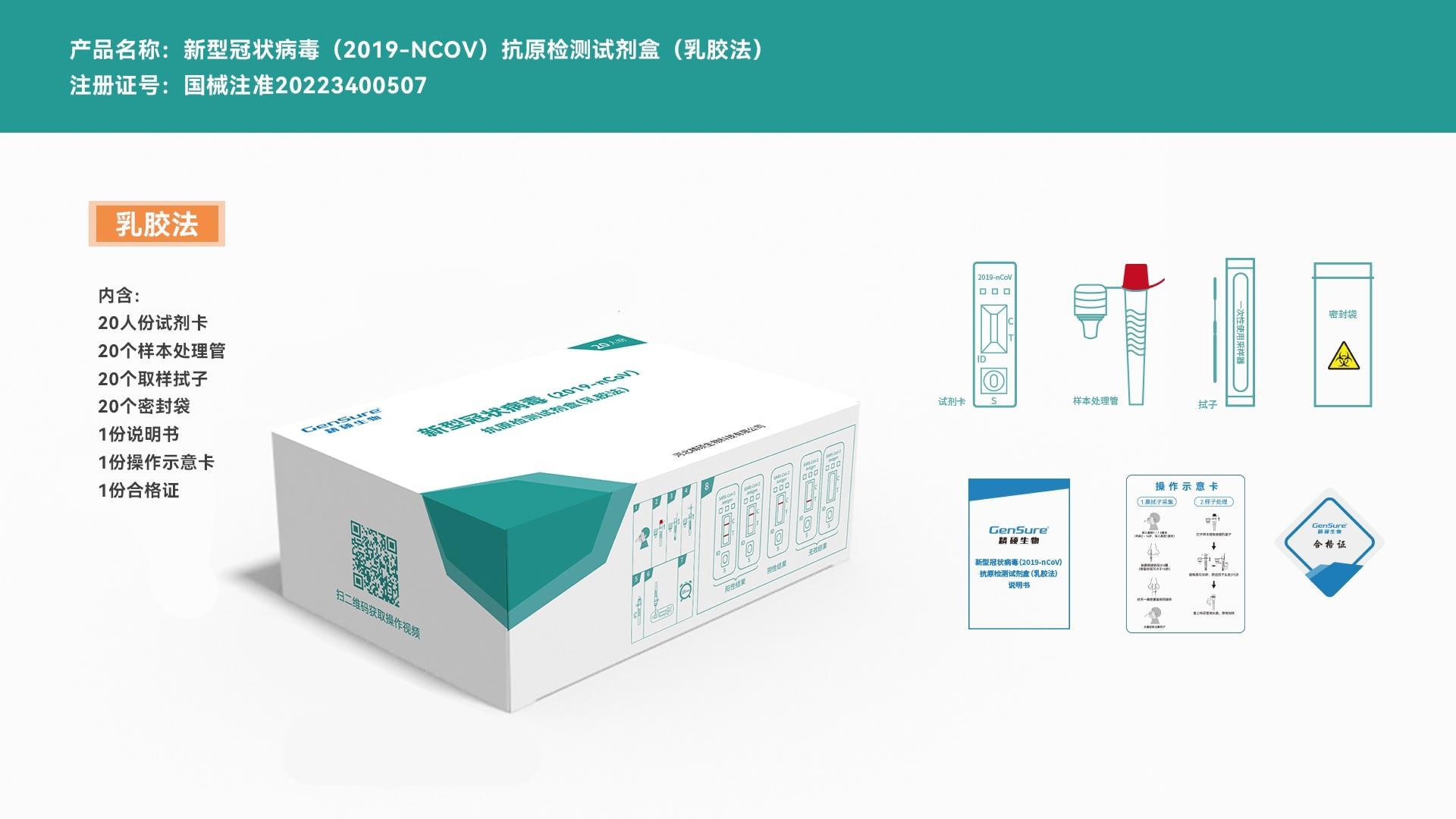
Product Content
[Product Name]Novel Coronavirus (2019-nCoV) Antigen Detection Kit (Latex Method)
[Registration Certificate Number]National Medical Device Approval 20223400507
[Packaging Specifications]1 person/bag, 5 persons/bag, 10 persons/bag, 20 persons/bag, 1 person/box, 5 persons/box, 10 persons/box, 20 persons/box, 25 persons/box, 30 persons/box, 40 persons/box, 50 persons/box, 100 persons/box, 200 persons/box.
[Intended Use]This product is used for the qualitative detection of the novel coronavirus (2019-nCoV) N protein antigen in human nasal swab samples. The applicable population should follow the national regulations such as the "Application Plan for Novel Coronavirus Antigen Detection (Trial)".
[Testing Principle]This product uses latex immunochromatography technology to detect the novel coronavirus (2019-nCoV) N protein antigen in human nasal swabs using a double antibody sandwich method.
[Product Features]
* True early screening, shortening the window period
* Detects novel coronavirus infection earlier, results can be read in 15 minutes
* Good sampling comfort * Effectively detects multiple novel coronavirus variants
* Pre-packaged extraction solution, simplified operation
* Store in the dark at 4~30℃, with a shelf life of up to 18 months
Packaging Content
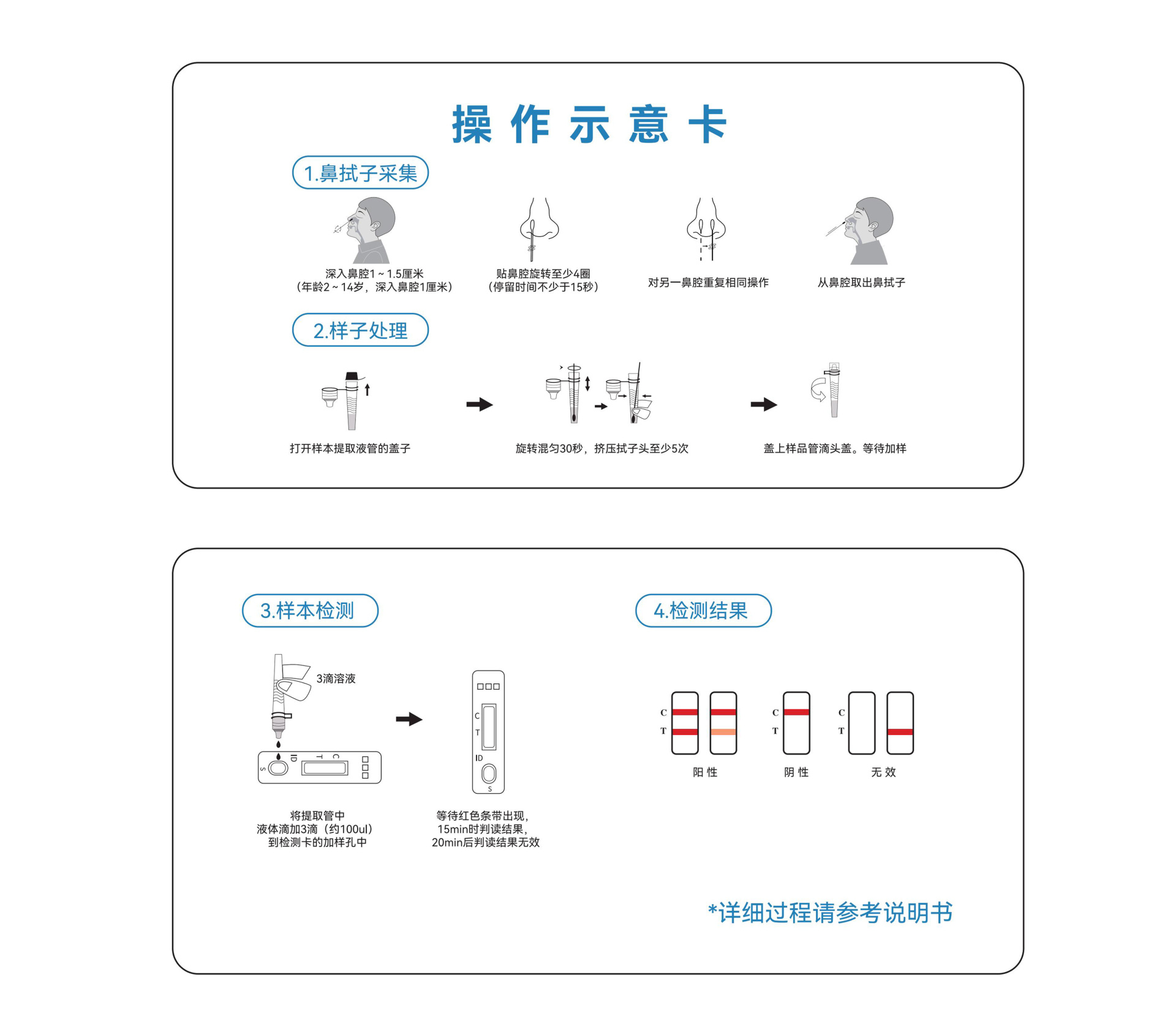
Operation Diagram
Product Specifications
|
Product Name |
Novel Coronavirus (2019-nCoV) Antigen Detection Kit |
Result Interpretation Time |
10-15 minutes |
|
Intended Use |
Novel Coronavirus (2019-nCoV) Antigen |
Is auxiliary reading equipment needed? |
No |
|
Sample Type |
Nasal Swab |
Storage Temperature |
4-30°C |
|
Certificate |
Medical Device Registration Certificate issued by the National Medical Products Administration |
Shelf Life |
18 months |
|
Packaging
|
1 person/bag, 5 persons/bag, 10 persons/bag, 20 persons/bag, 1 person/box, 5 persons/box, 10 persons/box, 20 persons/box, 25 persons/box, 30 persons/box, 40 persons/box, 50 persons/box, 100 persons/box, 200 persons/box. |
||
Product Packaging
Novel Coronavirus (2019-nCoV) Detection Kit - 1 Person
Novel Coronavirus (2019-nCoV) Detection Kit - 20X1

Novel Coronavirus (2019-nCoV) Detection Kit - 1 Person Soft Package

Novel Coronavirus (2019-nCoV) Detection Kit - 1 Person Soft Package

Novel Coronavirus (2019-nCoV) Detection Kit - 2 Persons

Novel Coronavirus (2019-nCoV) Detection Kit - 20X2
Novel Coronavirus (2019-nCoV) Detection Kit - 2 Persons

Novel Coronavirus (2019-nCoV) Detection Kit - 2 Persons

Novel Coronavirus (2019-nCoV) Detection Kit - 5 Persons - Soft Package

Novel Coronavirus (2019-nCoV) Detection Kit - 10 Persons

Novel Coronavirus (2019-nCoV) Detection Kit - 20 Persons

Scan the QR code to get the operation video for the Novel Coronavirus (2019-nCoV) Antigen Rapid Detection Kit
immediate consultation
If you are interested in our products, please leave your email, we will contact you as soon as possible, thank you!






