Good News! GenSure FOB-Fecal Occult Blood Rapid Test Approved by MOH Indonesia
We are delighted to announce that the GenSure FOB-Fecal Occult Blood Rapid Test has successfully obtained the Ministry of Health (MOH) Indonesia AKL Registration Certificate (KEMENKES RI AKL 20206520022).This certification marks another important milestone in our global market access, providing strong support for entering the Indonesian market.


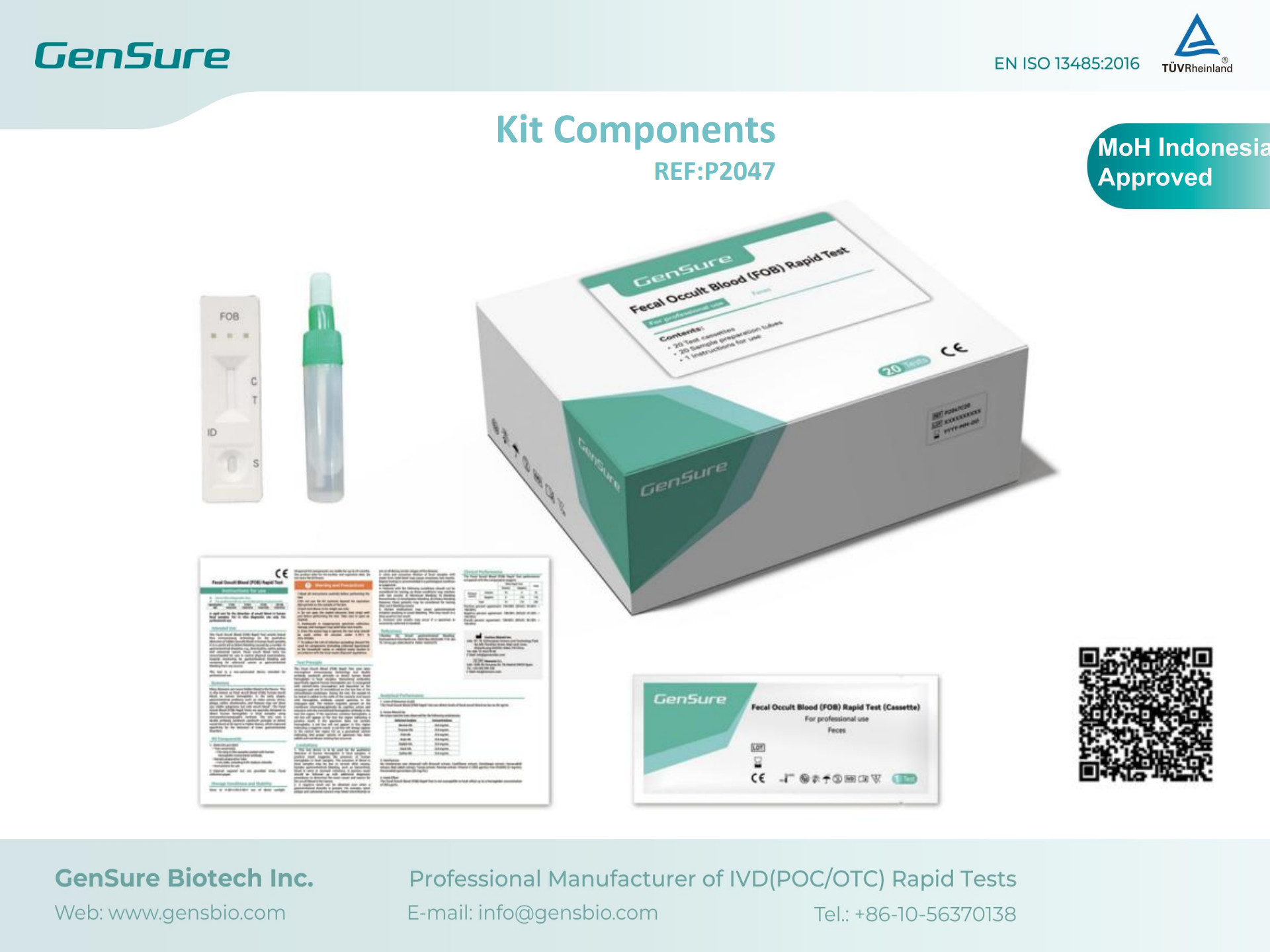
The GenSure FOB Rapid Test is designed for the qualitative detection of fecal occult blood, offering clinicians a simple, fast, and reliable diagnostic tool, particularly useful for the early screening of gastrointestinal bleeding and colorectal cancer.
The certificate is valid until June 23, 2030, laying a solid foundation for our sustainable development in Indonesia and the broader Southeast Asian market. We will continue to uphold our commitment to innovation, professionalism, and quality, delivering high-standard in vitro diagnostic products to users worldwide.

About Us
GenSure Biotech Inc. is a high-tech enterprise dedicated to the research, development, manufacturing, and sales of in vitro diagnostic (IVD) products. Our portfolio covers respiratory, gastrointestinal, infectious diseases, cancer screening, and chronic disease management. With registrations in dozens of countries and regions worldwide, our products are trusted and widely applied in global healthcare.
Contact Us
🌐 Website: www.gensbio.com
📧 Email: info@gensbio.com
📞 Tel: +86-10-56370138
[Beijing, China — Date] – GenSure Biotech Inc. today reached a significant milestone as four of its at-home rapid diagnostic test kits — Strep A, LH Ovulation, HCG Pregnancy, and Flu A+B — have simultaneously obtained EU CE IVDR certification. This achievement not only serves as solid proof of product excellence but also marks a critical step forward in the company’s globalization strategy.
2025-08-24
Good News! GenSure FOB-Fecal Occult Blood Rapid Test Approved by MOH Indonesia
We are delighted to announce that the GenSure FOB-Fecal Occult Blood Rapid Test has successfully obtained the Ministry of Health (MOH) Indonesia AKL Registration Certificate (KEMENKES RI AKL 20206520022).
2025-08-20
2024-11-04
2025-09-13
GenSure Invites you to PHARMED & HEALTHCARE VIETNAM
GenSure Invites you to PHARMED & HEALTHCARE VIETNAM
2025-09-13






