GenSure Biotech Secures CE IVDR Certification for Four At-Home Rapid Test Kits, Marking a New Chapter in Globalization Strategy
[Beijing, China — 2025.8.22] – GenSure Biotech Inc. today reached a significant milestone as four of its at-home rapid diagnostic test kits — Strep A, LH Ovulation, HCG Pregnancy, and Flu A+B — have simultaneously obtained EU CE IVDR certification. This achievement not only serves as solid proof of product excellence but also marks a critical step forward in the company’s globalization strategy.
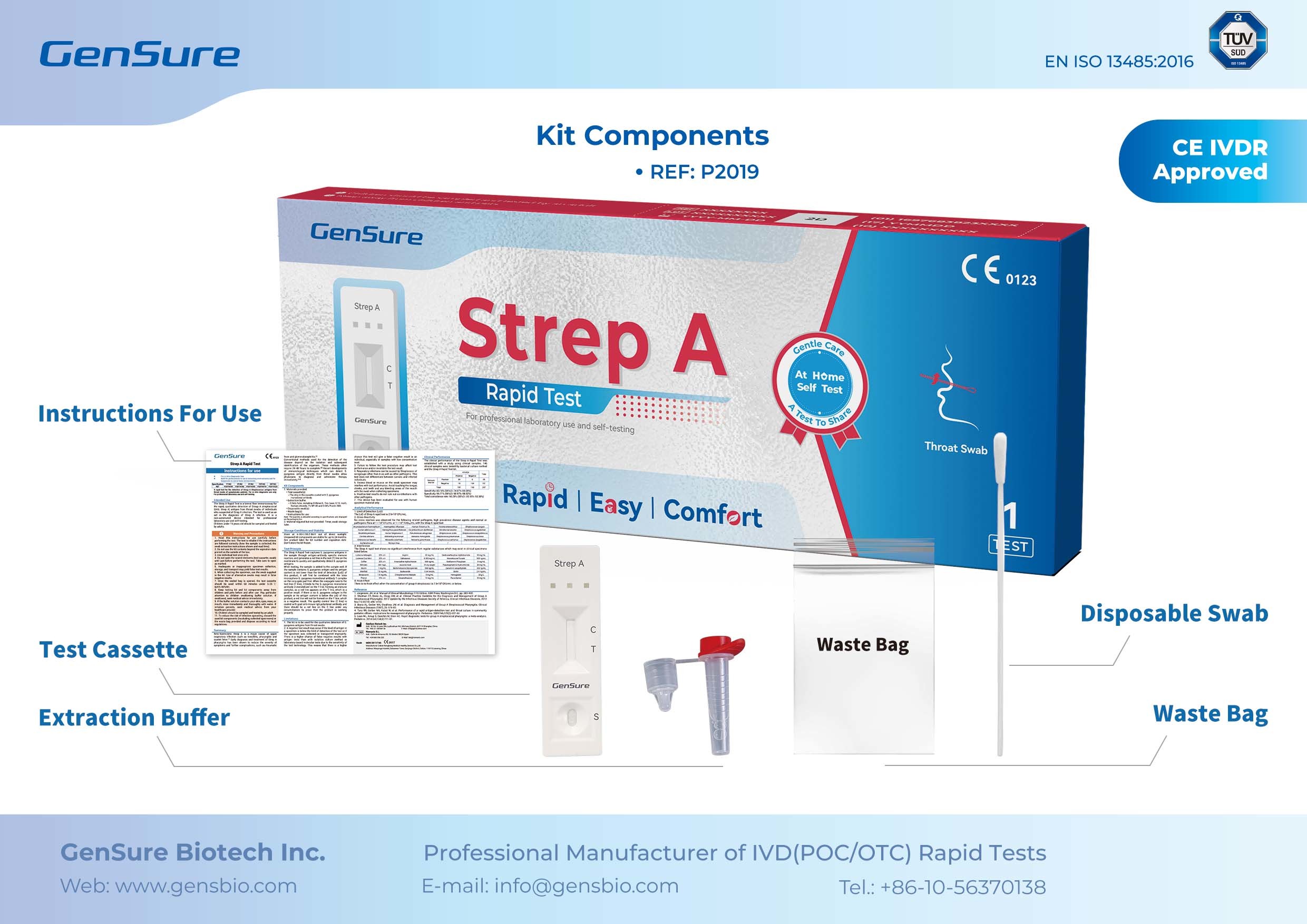
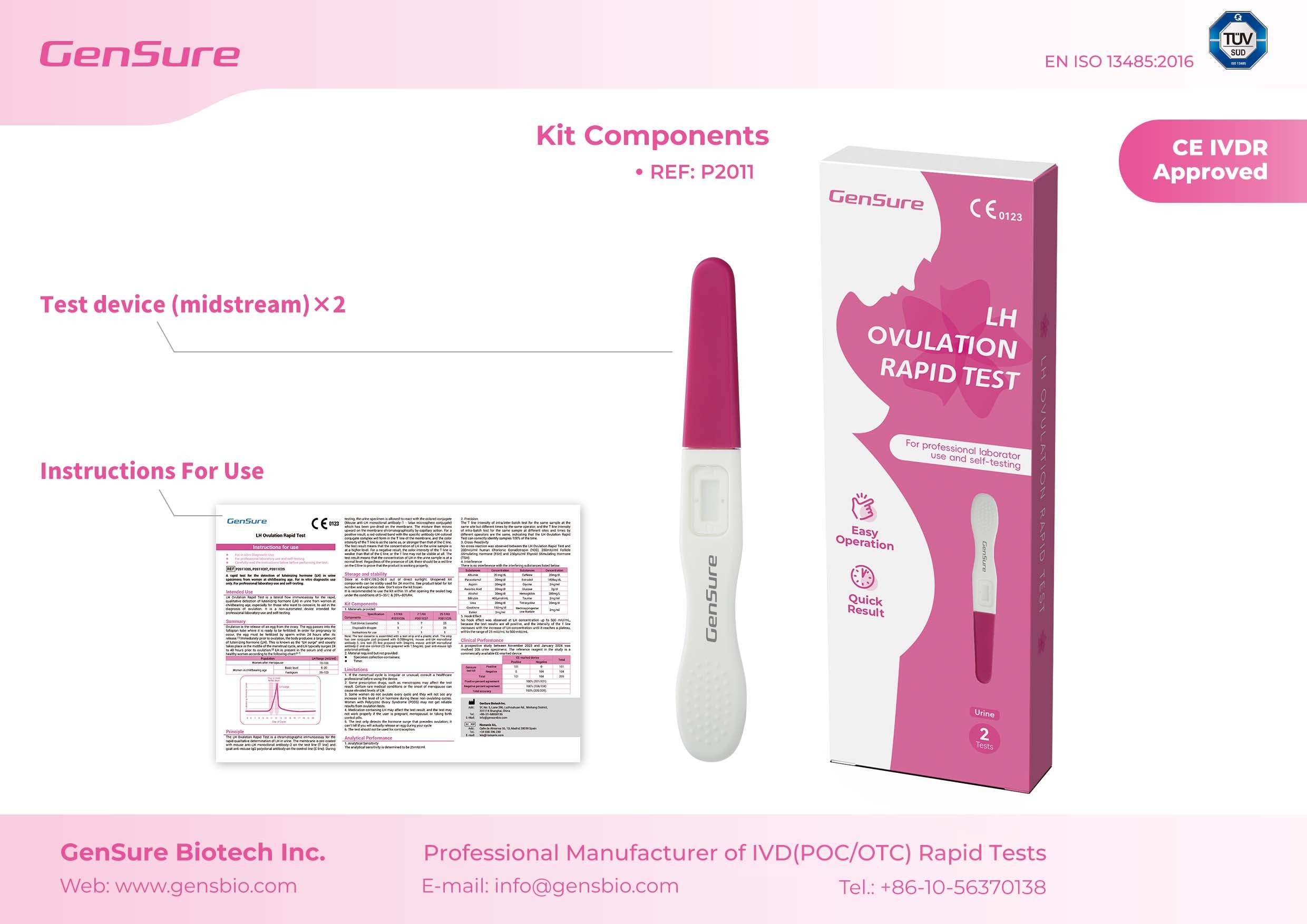



1. Superior Quality Opens the Door to International Markets
The certification process, which spanned nearly two years, involved the establishment of a cross-functional task force and multi-million RMB investment. From R&D optimization to clinical validation, every stage adhered strictly to international standards. By utilizing internationally recognized reference materials for repeated verification, the tests demonstrated sensitivity, specificity, and performance that fully meet EU requirements. Independent clinical evaluations conducted at European centers further confirmed their reliability for both home and professional use.
Strep A test helps reduce antibiotic misuse.
Flu A+B test enables rapid influenza typing.
LH and HCG tests provide accurate support for fertility and pregnancy monitoring.
These four flagship products, with their outstanding performance, have earned an international “passport” for market entry.
2. Building a Solid Foundation for Global Expansion
Obtaining the CE IVDR certification represents a key milestone in GenSure Biotech’s globalization roadmap. As the EU’s latest and most stringent medical device regulation, IVDR certification signifies that the company’s product quality, manufacturing systems, and clinical performance are now fully aligned with international standards — significantly enhancing trust among global customers.
This achievement not only clears the path for entry into the European market but also opens broader global opportunities, laying a solid foundation for more products to go international. It underscores GenSure Biotech’s commitment to advancing global public health through innovation and excellence in diagnostics.
About Us
GenSure Biotech Inc. is a high-tech enterprise specializing in the research, development, manufacturing, and commercialization of in vitro diagnostic (IVD) products. Our portfolio covers multiple fields, including respiratory, gastrointestinal, infectious diseases, cancer screening, and chronic disease management. GenSure products have been registered and widely adopted in dozens of countries and regions worldwide.
Contact Us
Website: www.gensbio.com
Email: info@gensbio.com
Tel: +86-10-56370138
[Beijing, China — Date] – GenSure Biotech Inc. today reached a significant milestone as four of its at-home rapid diagnostic test kits — Strep A, LH Ovulation, HCG Pregnancy, and Flu A+B — have simultaneously obtained EU CE IVDR certification. This achievement not only serves as solid proof of product excellence but also marks a critical step forward in the company’s globalization strategy.
2025-08-24
Good News! GenSure FOB-Fecal Occult Blood Rapid Test Approved by MOH Indonesia
We are delighted to announce that the GenSure FOB-Fecal Occult Blood Rapid Test has successfully obtained the Ministry of Health (MOH) Indonesia AKL Registration Certificate (KEMENKES RI AKL 20206520022).
2025-08-20
2024-11-04
2025-09-13
GenSure Invites you to PHARMED & HEALTHCARE VIETNAM
GenSure Invites you to PHARMED & HEALTHCARE VIETNAM
2025-09-13






